Brilliant Strategies Of Tips About How To Write Combustion Reactions

A combustion reaction is a reaction between a fuel and oxidizer to form an oxidized product.
How to write combustion reactions. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. It contains plenty of examples and practice. Writing equations for combustion reactions chemistry.
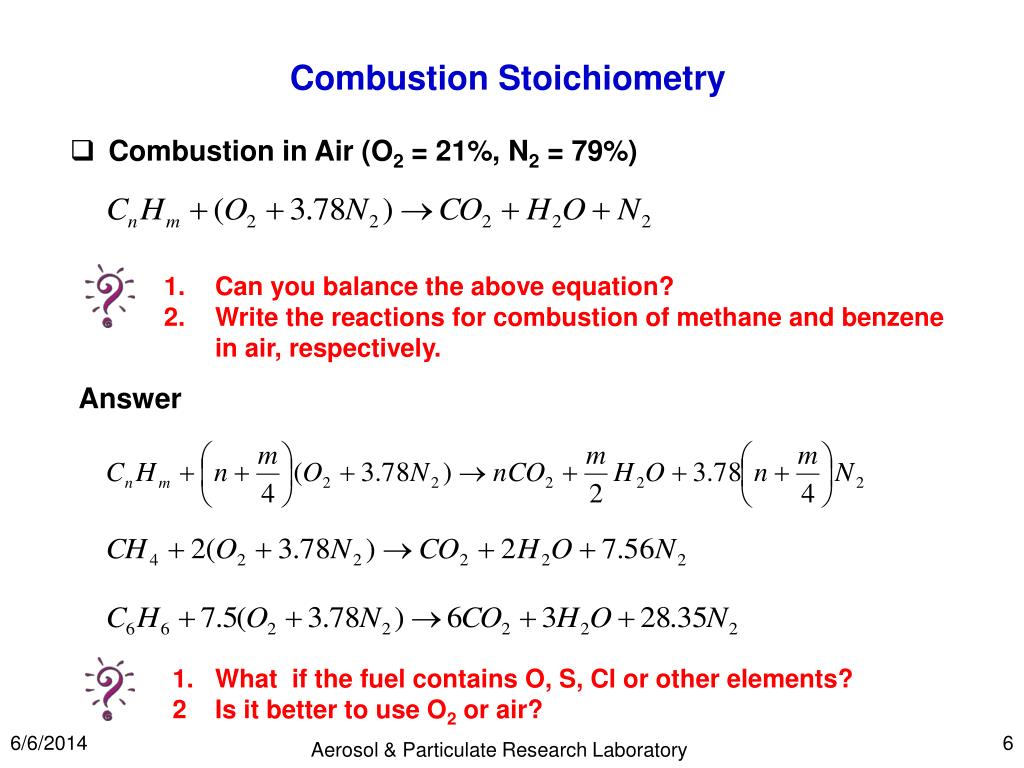
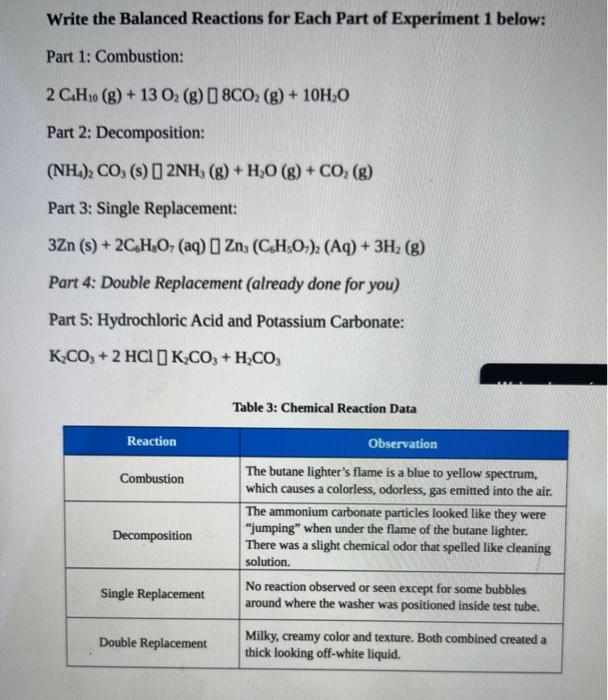
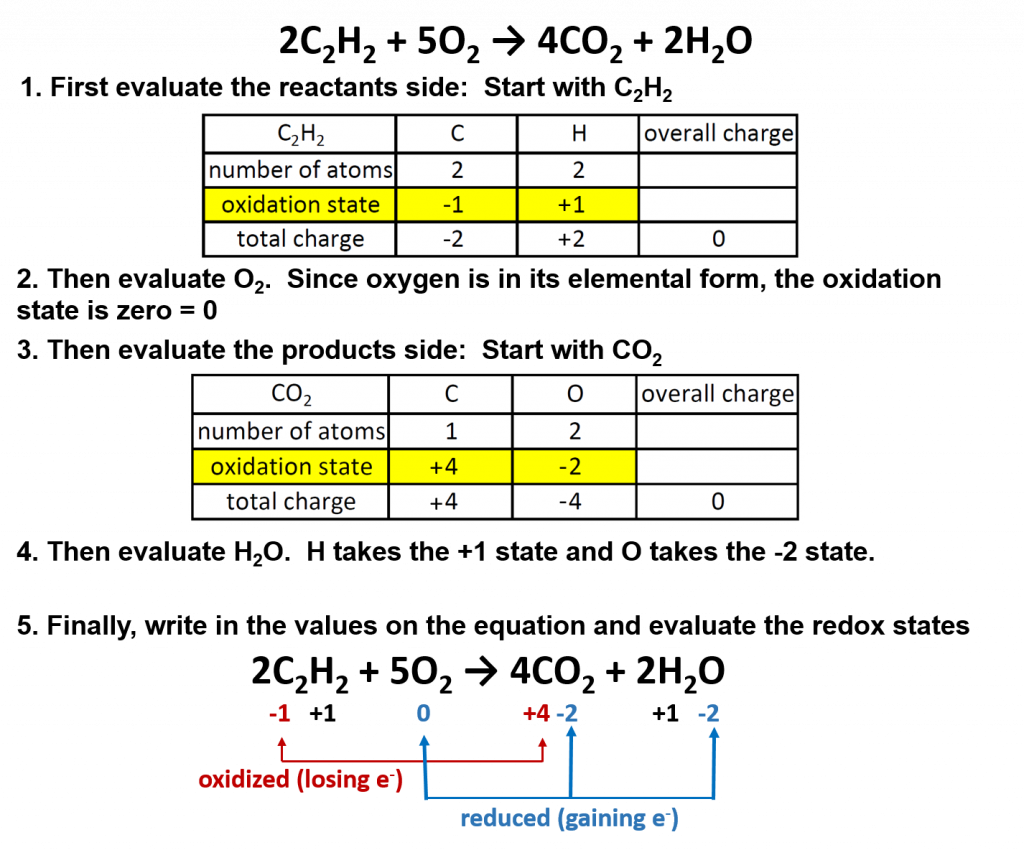
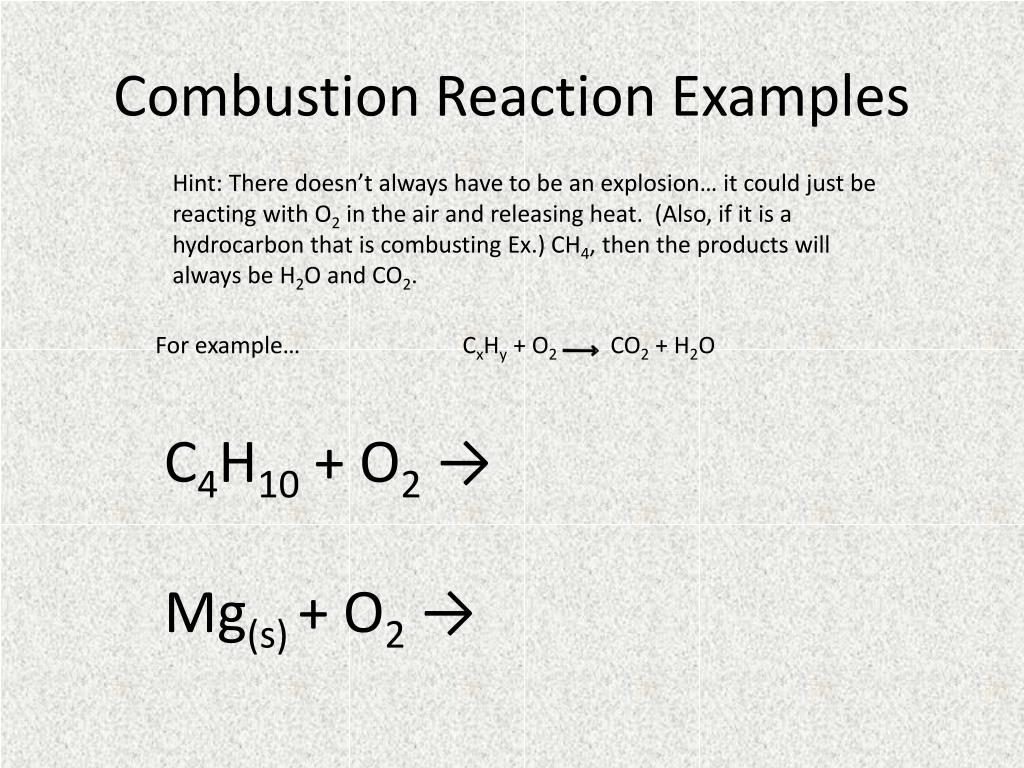
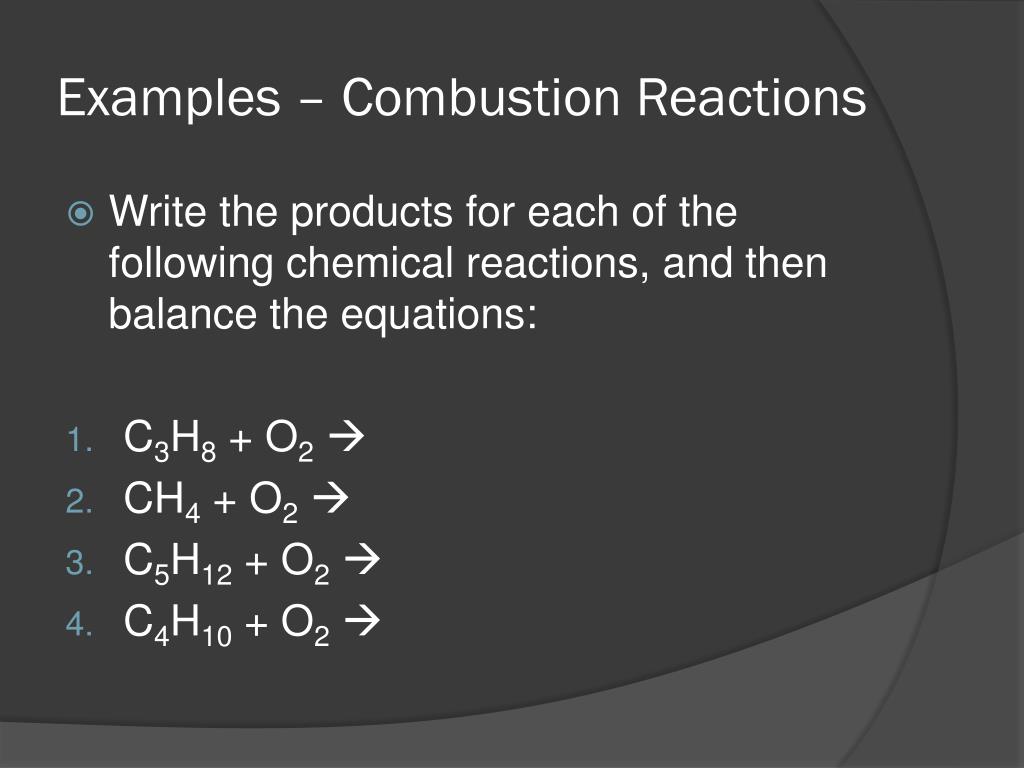
A general way to write a combustion reaction is. However, we will understand combustion to mean the reaction of oxygen with an. 2 c 2 h 6 + 7 o 2 → 4 co 2 + 6 h 2 o.
An example of a combustion. Combustion, at its most general, can mean the reaction of oxygen gas (o 2) with anything. Combustion reactions must involve o2 o 2 as one reactant.
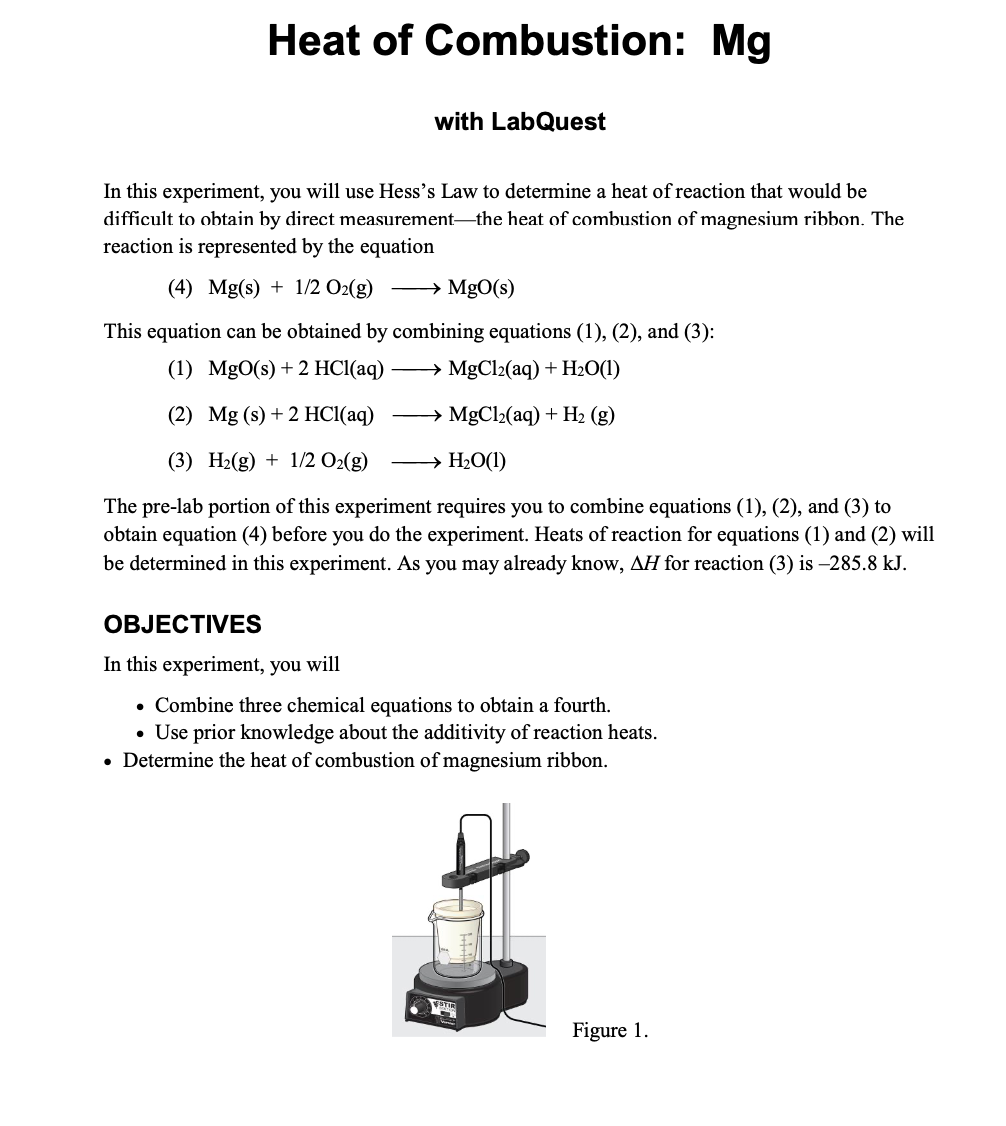
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a kind of chemical reaction in which a reaction between any combustible substance and an oxidiser takes place in order to form an oxidised product. In a combustion reaction, a.
Usually, a hydrocarbon reacts with oxygen to form carbon dioxide and. 436k views 6 years ago. Combustion reactions involve o 2 as one.
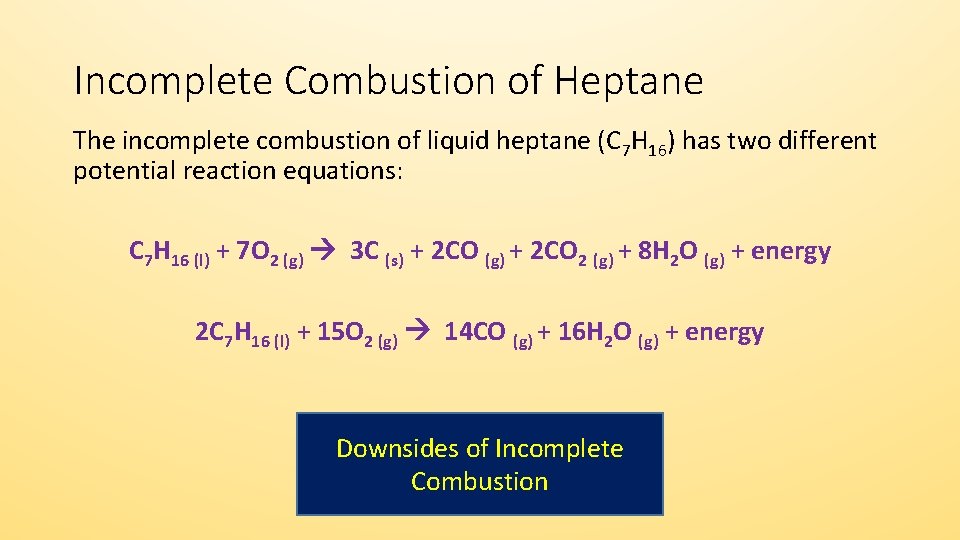
The complete combustion of any hydrocarbon with sufficient oxygen always yields carbon dioxide and water. This video goes through how to w. What is a combustion reaction?
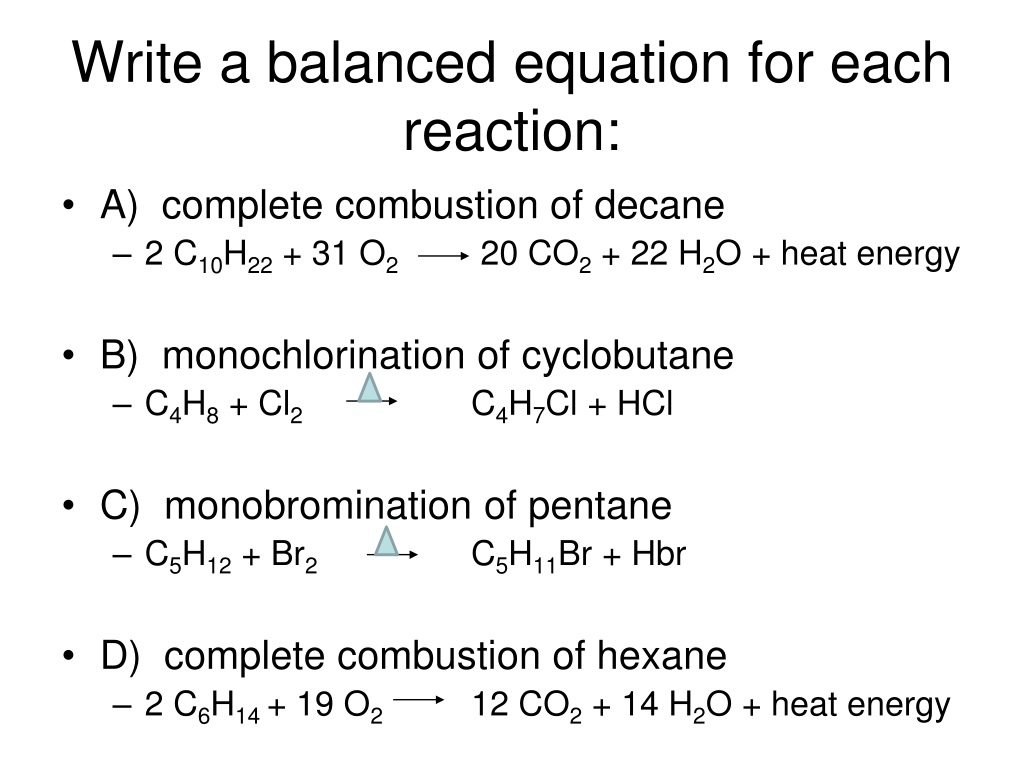
Describe some of the characteristics of. Combustion is another word for burning. A balanced chemical equation shows the same number of each type of atom on both sides of the arrow.
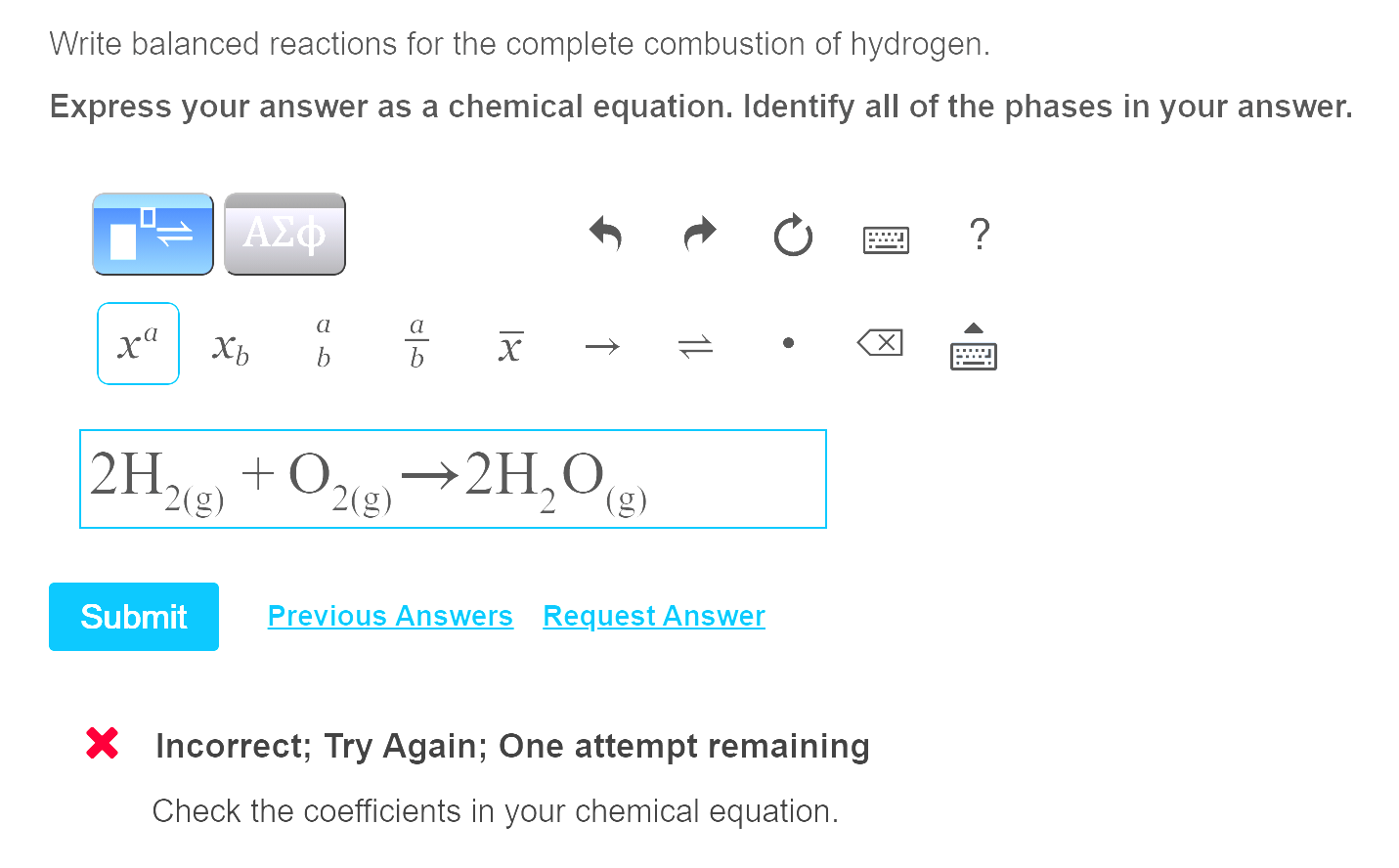
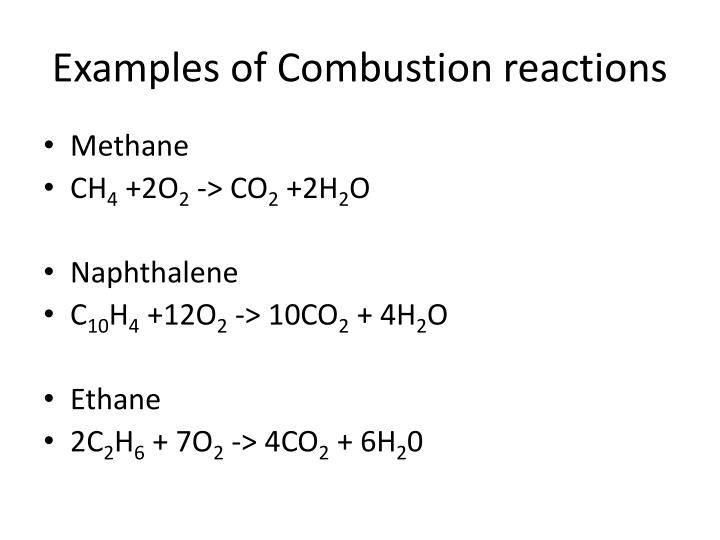
C 10 h 8 + 12 o 2 → 10 co 2 + 4 h 2 o. The combustion of hydrogen gas produces water vapor:. Identify a general chemical equation for combustion reactions.
What are the three parts of the fire triangle? This is a chemistry tutorial lesson on how to write a combustion reaction, or reaction where a hydrocarbon is burned in air. Burning of naphthalene.
In this example, we balance the combustion reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A decomposition reaction produces multiple products from a single reactant.
/methanecombustion-58e3e6005f9b58ef7e0daa10.jpg)





/methanecombustion-58e3e6005f9b58ef7e0daa10.jpg)